Cytoskeletal Priming of Mesenchymal Stem Cells to a Medicinal Phenotype
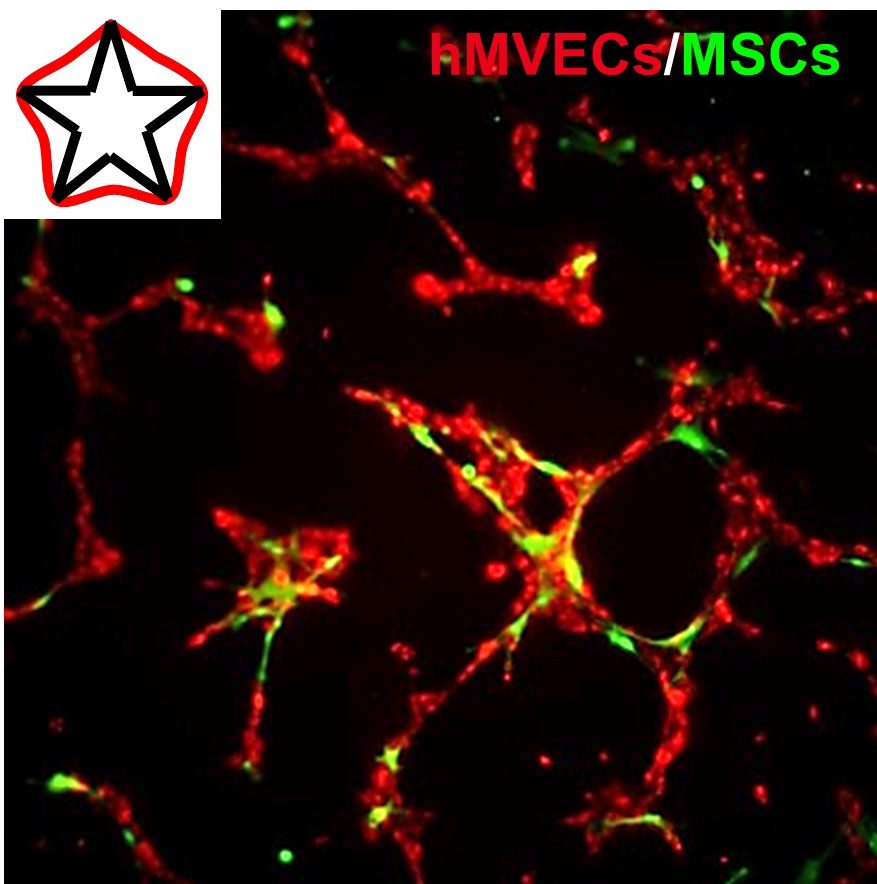
Mesenchymal stem cell (MSC) therapy is a promising approach for the treatment of cardiovascular disease, demonstrating pronounced trophic, immunomodulatory, and pro-angiogenic activity. However, clinical efficacy has suffered from broad variability, presumably due to cell death upon implantation, and the heterogeneous population of autologous cells. Micropatterning single cells in the same geometry can normalize the phenotype in a population, and variations in subcellular curvature will guide focal adhesion, cytoskeletal organization, and the regulation of distinct epigenetic marks to orchestrate a medicinal secretome. Within 2 days, activated cells show elevated expression of pericyte markers and will recapitulate functional pericyte activity through enhanced association with endothelial cell tubules in co-culture. MSCs are believed to undergo a temporary switch in vivo to an activated state in response to injury; thus, we propose engineering actomyosin contractility after isolation can similarly activate MSCs, which may serve as a general approach to prime a medicinal phenotype for cell-based therapies.