Matrix Mechanics Influence Fibroblast–Myofibroblast Transition by Directing the Localization of Histone Deacetylase 4
Introduction—The propagation of mechanochemical signals from the extracellular matrix to the cell nucleus has emerged as a central feature in regulating cellular differentiation and de-differentiation. This process of outside-in signaling and the associated mechanotransduction pathways have been well described in numerous developmental and pathological contexts. However, it remains less clear how mechanotransduction influences the activity of chromatin modifying enzymes that direct gene expression programs.
Objectives—The primary objective of this study was to explore how matrix mechanics and geometric confinement influence histone deacetylase (HDAC) activity in fibroblast culture. Methods—Polyacrylamide hydrogels were formed and functionalized with fibronectin patterns using soft lithography. Primary mouse embryonic fibroblasts (MEFs) were cultured on the islands until confluent, fixed, and immunolabeled for microscopy.
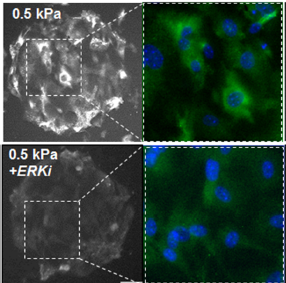
Results—After 24 h MEFs cultured on soft hydrogels (0.5 kPa) show increased expression of class I HDACs relative to MEFs cultured on stiff hydrogels (100 kPa). A member of the class II family, HDAC4 shows a similar trend; however, there is a pronounced cytoplasmic localization on soft hydrogels suggesting a role in outside-in cytoplasmic signaling. Pharmacological inhibitor studies suggest that the opposing activities of extracellular related kinase 1/2 (ERK) and protein phosphatase 2a (PP2a) influence the localization of HDAC4. MEFs cultured on the soft hydrogels show enhanced expression of markers associated with a fibroblast– myofibroblast transition (Paxillin, aSMA).
Conclusions—We show that the phosphorylation state and cellular localization of HDAC4 is influenced by matrix mechanics, with evidence for a role in mechanotransduction and the regulation of gene expression associated with fibroblast–myofibroblast transitions. This work establishes a link between outside-in signaling and epigenetic regulation, which will assist efforts aimed at controlling gene regulation in engineered extracellular matrices.