Vertical Integration of Cell-Laden Hydrogels with Bioinspired Photonic Crystal Membranes
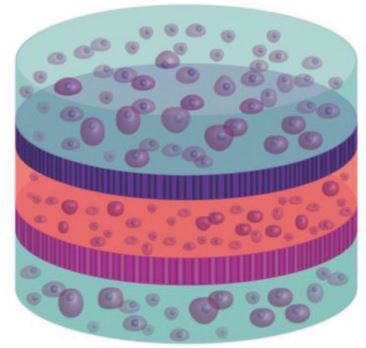
The native architecture of tissue segregates populations of cells through nanostructured membranes of biopolymers (e.g., basement membranes) which serve the dual purpose of providing scaffolding for cells and filtration through size‐exclusion. A multilayered approach to fabricate tissue scaffolds where populations of encapsulated cells are separated by porous silicon (PSi)‐based photonic crystal membranes with nano‐ and microstructure that mimics the basement membranes is reported here. The PSi films are fabricated to display discreet photonic bandgaps such that remote optical interrogation provides a specific resonance for each film. Through careful control of surface chemistry, nanostructured PSi films are engineered to serve as optical biosensors and biomolecule release reservoirs, where the optical signature can report on these distinct functions remotely using a simple light source. The promise of this approach is demonstrated as a “smart” tissue scaffolding by monitoring matrix metalloprotease (MMP) activity from encapsulated multilayered co‐cultures of mesenchymal stem cells and human microvascular endothelial cells, with concurrent attenuation of MMP activity through release of angiogenic‐modulating compounds. The integration of optically registered biosensing and drug‐release capabilities—within a multilayered hydrogel scaffold with multiple cell types—provides a new approach to tissue engineering where dynamic bioactivity can be monitored remotely in real‐time.