Bioengineering
Publication Types:
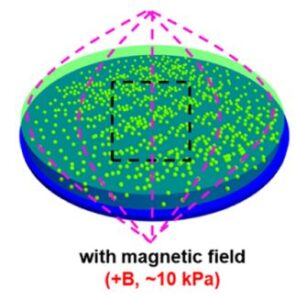
Gradient and Dynamic Hydrogel Materials to Probe Dynamics in Cancer Stem Cell Phenotypes
The microenvironment of tumors shows high variability in stiffness compared to normal tissues, suggesting that spatiotemporal changes in mechanics play a role in development and progression. Here, we employ microengineered hydrogels with static, dynamic (magnetic field-mediated stiffening and softening), or gradient matrix elasticity to investigate the influence of matrix mechanics in modulating the stem cell-like phenotype in melanoma and breast cancer cells. Using immunofluorescence staining of molecular markers associated with a cancer stem cell (CSC) state, we show that a subtle increase in local stiffness promotes the CSC phenotype, while different mechanical properties in static or dynamic hydrogelswithout gradient profiles of mechanical propertieshave a negligible influence on phenotype switching toward a CSC state. Inhibition of integrins and downstream effectors of mechanotransduction reveals that distinct signaling pathways play a role in regulating the dynamic CSC state in melanoma and breast cancer cells. Our findings demonstrate how cancer cells respond to the local stiffness gradients with dynamic plasticity during progression.
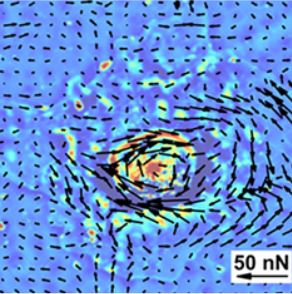
Simultaneous cell traction and growth measurements using light
Characterizing the effects of force fields generated by cells on proliferation, migration and differentiation processes is challenging due to limited availability of nondestructive imaging modalities. Here, we integrate a new real‐time traction stress imaging modality, Hilbert phase dynamometry (HPD), with spatial light interference microscopy (SLIM) for simultaneous monitoring of cell growth during differentiation processes. HPD uses holographic principles to extract displacement fields from chemically patterned fluorescent grid on deformable substrates. This is converted into forces by solving an elasticity inverse problem. Since HPD uses the epi‐fluorescence channel of an inverted microscope, cellular behavior can be concurrently studied in transmission with SLIM. We studied the differentiation of mesenchymal stem cells (MSCs) and found that cells undergoing osteogenesis and adipogenesis exerted larger and more dynamic stresses than their precursors, with MSCs developing the smallest forces and growth rates. Thus, we develop a powerful means to study mechanotransduction during dynamic processes where the matrix provides context to guide cells toward a physiological or pathological outcome.
Quantitative phase imaging reveals matrix stiffness-dependent growth and migration of cancer cells.
Cancer progression involves complex signals within the tumor microenvironment that orchestrate proliferation and invasive processes. The mechanical properties of the extracellular matrix (ECM) within this microenvironment has been demonstrated to influence growth and the migratory phenotype that precedes invasion. Here we present the integration of a label-free quantitative phase imaging technique, spatial light interference microscopy (SLIM)—with protein-conjugated hydrogel substrates— to explore how the stiffness of the ECM influences melanoma cells of varying metastatic potential. Melanoma cells of high metastatic potential demonstrate increased growth and velocity characteristics relative to cells of low metastatic potential. Cell velocity in the highly metastatic population shows a relative insensitivity to matrix stiffness suggesting adoption of migratory routines that are independent of mechanics to facilitate invasion. The use of SLIM and engineered substrates provides a new approach to characterize the invasive properties of live cells as a function of microenvironment parameters. This work provides fundamental insight into the relationship between growth, migration and metastatic potential, and provides a new tool for profiling cancer cells for clinical grading and development of patient-specific therapeutic regimens.

Effects of substrate patterning on cellular spheroid growth and dynamics measured by gradient light interference microscopy (GLIM)
The development of three-dimensional (3D) cellular architectures during development and pathological processes involves intricate migratory patterns that are modulated by genetics and the surrounding microenvironment. The substrate composition of cell cultures has been demonstrated to influence growth, proliferation and migration in 2D. Here, we study the growth and dynamics of mouse embryonic fibroblast cultures patterned in a tissue sheet which then exhibits 3D growth. Using gradient light interference microscopy (GLIM), a label-free quantitative phase imaging approach, we explored the influence of geometry on cell growth patterns and rotational dynamics. We apply, for the first time to our knowledge, dispersion-relation phase spectroscopy (DPS) in polar coordinates to generate the radial and rotational cell mass-transport. Our data show that cells cultured on engineered substrates undergo rotational transport in a radially independent manner and exhibit faster vertical growth than the control, unpatterned cells. The use of GLIM and polar DPS provides a novel quantitative approach to studying the effects of spatially patterned substrates on cell motility and growth.
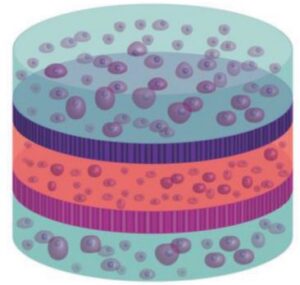
Vertical Integration of Cell-Laden Hydrogels with Bioinspired Photonic Crystal Membranes
The native architecture of tissue segregates populations of cells through nanostructured membranes of biopolymers (e.g., basement membranes) which serve the dual purpose of providing scaffolding for cells and filtration through size‐exclusion. A multilayered approach to fabricate tissue scaffolds where populations of encapsulated cells are separated by porous silicon (PSi)‐based photonic crystal membranes with nano‐ and microstructure that mimics the basement membranes is reported here. The PSi films are fabricated to display discreet photonic bandgaps such that remote optical interrogation provides a specific resonance for each film. Through careful control of surface chemistry, nanostructured PSi films are engineered to serve as optical biosensors and biomolecule release reservoirs, where the optical signature can report on these distinct functions remotely using a simple light source. The promise of this approach is demonstrated as a “smart” tissue scaffolding by monitoring matrix metalloprotease (MMP) activity from encapsulated multilayered co‐cultures of mesenchymal stem cells and human microvascular endothelial cells, with concurrent attenuation of MMP activity through release of angiogenic‐modulating compounds. The integration of optically registered biosensing and drug‐release capabilities—within a multilayered hydrogel scaffold with multiple cell types—provides a new approach to tissue engineering where dynamic bioactivity can be monitored remotely in real‐time.
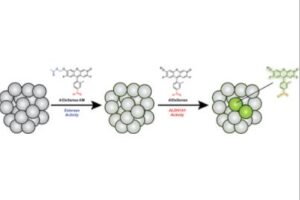
Surveillance of Cancer Stem Cell Plasticity Using an Isoform-Selective Fluorescent Probe for Aldehyde Dehydrogenase 1A1
Cancer stem cells (CSCs) are progenitor cells that contribute to treatment-resistant phenotypes during relapse. CSCs exist in specific tissue microenvironments that cell cultures and more complex models cannot mimic. Therefore, the development of new approaches that can detect CSCs and report on specific properties (e.g., stem cell plasticity) in their native environment have profound implications for studying CSC biology. Herein, we present AlDeSense, a turn-on fluorescent probe for aldehyde dehydrogenase 1A1 (ALDH1A1) and Ctrl-AlDeSense, a matching nonresponsive reagent. Although ALDH1A1 contributes to the detoxification of reactive aldehydes, it is also associated with stemness and is highly elevated in CSCs. AlDeSense exhibits a 20-fold fluorescent enhancement when treated with ALDH1A1. Moreover, we established that AlDeSense is selective against a panel of common ALDH isoforms and exhibits exquisite chemostability against a collection of biologically relevant species. Through the application of surface marker antibody staining, tumorsphere assays, and assessment of tumorigenicity, we demonstrate that cells exhibiting high AlDeSense signal intensity have properties of CSCs. Using these probes in tandem, we have identified CSCs at the cellular level via flow cytometry and confocal imaging, as well as monitored their states in animal models.
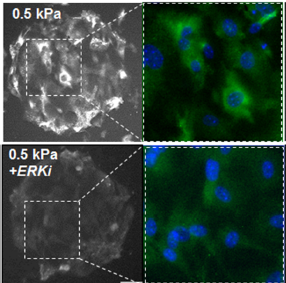
Matrix Mechanics Influence Fibroblast–Myofibroblast Transition by Directing the Localization of Histone Deacetylase 4
Introduction—The propagation of mechanochemical signals from the extracellular matrix to the cell nucleus has emerged as a central feature in regulating cellular differentiation and de-differentiation. This process of outside-in signaling and the associated mechanotransduction pathways have been well described in numerous developmental and pathological contexts. However, it remains less clear how mechanotransduction influences the activity of chromatin modifying enzymes that direct gene expression programs.
Objectives—The primary objective of this study was to explore how matrix mechanics and geometric confinement influence histone deacetylase (HDAC) activity in fibroblast culture. Methods—Polyacrylamide hydrogels were formed and functionalized with fibronectin patterns using soft lithography. Primary mouse embryonic fibroblasts (MEFs) were cultured on the islands until confluent, fixed, and immunolabeled for microscopy.
Results—After 24 h MEFs cultured on soft hydrogels (0.5 kPa) show increased expression of class I HDACs relative to MEFs cultured on stiff hydrogels (100 kPa). A member of the class II family, HDAC4 shows a similar trend; however, there is a pronounced cytoplasmic localization on soft hydrogels suggesting a role in outside-in cytoplasmic signaling. Pharmacological inhibitor studies suggest that the opposing activities of extracellular related kinase 1/2 (ERK) and protein phosphatase 2a (PP2a) influence the localization of HDAC4. MEFs cultured on the soft hydrogels show enhanced expression of markers associated with a fibroblast– myofibroblast transition (Paxillin, aSMA).
Conclusions—We show that the phosphorylation state and cellular localization of HDAC4 is influenced by matrix mechanics, with evidence for a role in mechanotransduction and the regulation of gene expression associated with fibroblast–myofibroblast transitions. This work establishes a link between outside-in signaling and epigenetic regulation, which will assist efforts aimed at controlling gene regulation in engineered extracellular matrices.
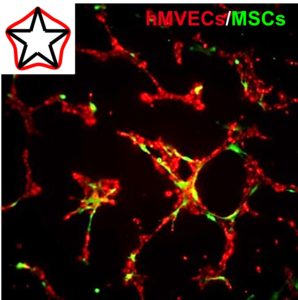
Cytoskeletal Priming of Mesenchymal Stem Cells to a Medicinal Phenotype
Mesenchymal stem cell (MSC) therapy is a promising approach for the treatment of cardiovascular disease, demonstrating pronounced trophic, immunomodulatory, and pro-angiogenic activity. However, clinical efficacy has suffered from broad variability, presumably due to cell death upon implantation, and the heterogeneous population of autologous cells. Micropatterning single cells in the same geometry can normalize the phenotype in a population, and variations in subcellular curvature will guide focal adhesion, cytoskeletal organization, and the regulation of distinct epigenetic marks to orchestrate a medicinal secretome. Within 2 days, activated cells show elevated expression of pericyte markers and will recapitulate functional pericyte activity through enhanced association with endothelial cell tubules in co-culture. MSCs are believed to undergo a temporary switch in vivo to an activated state in response to injury; thus, we propose engineering actomyosin contractility after isolation can similarly activate MSCs, which may serve as a general approach to prime a medicinal phenotype for cell-based therapies.
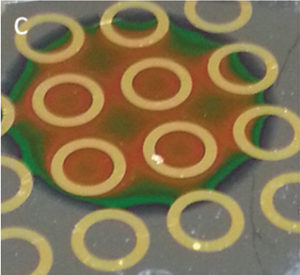
Patterned porous silicon photonic crystals with modular surface chemistry for spatial control of neural stem cell differentiation
We present a strategy to spatially define regions of gold and nanostructured silicon photonics, each with materials-specific surface chemistry, for azide–alkyne cycloaddition of different bioactive peptides. Neural stem cells are spatially directed to undergo neurogenesis and astrogenesis as a function of both surface properties and peptide identity.
Bridging the gap: from 2D cell culture to 3D microengineered extracellular matrices
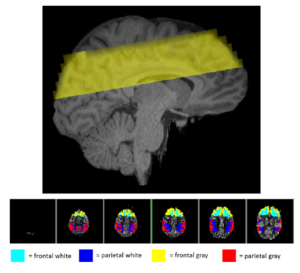
Cardiorespiratory fitness mediates the effects of aging on cerebral blood flow
The brain’s vasculature is likely to be subjected to the same age-related physiological and anatomical changes affecting the rest of the cardiovascular system. Since aerobic fitness is known to alleviate both cognitive and volumetric losses in the brain, it is important to investigate some of the possible mechanisms underlying these beneficial changes. Here we investigated the role that estimated cardiorespiratory fitness (eCRF) plays in determining the relationship between aging and cerebral blood flow (CBF) in a group of older adults (ages 55–85). Using arterial spin labeling to quantify CBF, we found that blood flow in the gray matter was positively correlated with eCRF and negatively correlated with age. Subsequent analyses revealed that eCRF fully mediated the effects of age on CBF in the gray matter, but not in the white matter. Additionally, regional measures of CBF were related to regional measures of brain volume. These findings provide evidence that age-related effects on cerebrovascular health and perfusion in older adults are largely influenced by their eCRF levels.
