Comparative Bioscience
Publication Types:
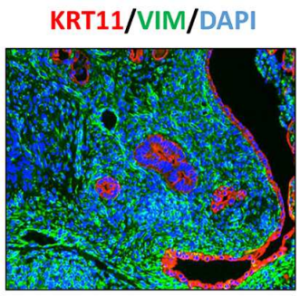
Progesterone Alleviates Endometriosis via Inhibition of Uterine Cell Proliferation, Inflammation and Angiogenesis in an Immunocompetent Mouse Model
Endometriosis, defined as growth of the endometrial cells outside the uterus, is an inflammatory disorder that is associated with chronic pelvic pain and infertility in women of childbearing age. Although the estrogen-dependence of endometriosis is well known, the role of progesterone in development of this disease remains poorly understood. In this study, we developed a disease model in which endometriosis was induced in the peritoneal cavities of immunocompetent female mice, and maintained with exogenous estrogen. The endometriosis-like lesions that were identified at a variety of ectopic locations exhibited abundant blood supply and extensive adhesions. Histological examination revealed that these lesions had a well-organized endometrial architecture and fibrotic response, resembling those recovered from clinical patients. In addition, an extensive proliferation, inflammatory response, and loss of estrogen receptor alpha (ERα) and progesterone receptor (PR) expression were also observed in these lesions. Interestingly, administration of progesterone before, but not after, lesion induction suppressed lesion expansion and maintained ERα and PR expressions. These progesterone-pretreated lesions exhibited attenuation in KI67, CD31, and pro-inflammatory cytokine expression as well as macrophage infiltration, indicating that progesterone ameliorates endometriosis progression by inhibiting cell proliferation, inflammation and neovascularization. Our studies further showed that suppression of global DNA methylation by application of DNA methyltransferase inhibitor to female mice bearing ectopic lesions restrained lesion expansion and restored ERα and PR expression in eutopic endometrium and ectopic lesions. These results indicate that epigenetic regulation of target gene expression via DNA methylation contributes, at least in part, to progesterone resistance in endometriosis.
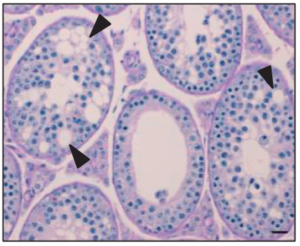
Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and Sertoli cells
Basigin (BSG) is a multifunctional glycoprotein that plays an important role in male reproduction since male knockout (KO) mice are sterile. The Bsg KO testis lacks elongated spermatids and mature spermatozoa, a phenotype similar to that of alpha-mannosidase IIx (MX) KO mice. MX regulates formation of N-acetylglucosamine (GlcNAc) terminated N-glycans that participate in germ cell–Sertoli cell adhesion. Results showed that Bsg KO spermatocytes displayed normal homologous chromosome synapsis and progression through meiosis. However, only punctate expression of the round spermatid marker SP-10 in the acrosomal granule of germ cells of Bsg KO mice was detected indicating that spermatogenesis in Bsg KO mice was arrested at the early round spermatid stages. We observed a large increase in the number of germ cells undergoing apoptosis in Bsg KO testes. Using lectin blotting, we determined that GlcNAc terminated N-glycans are linked to BSG. GlcNAc terminated N-glycans were significantly reduced in Bsg KO testes. These observations indicate that BSG may act as a germ cell-Sertoli cell attachment molecule. Loss of BSG significantly reduced adhesion between GC-2 and SF7 cells. Moreover, wild type testes showed strong expression of N-cadherin (CDH2) while expression was greatly reduced in the testes of Bsg KO mice. In addition, the integrity of the blood-testis barrier (BTB) was compromised in Bsg KO testes. In conclusion, although some Bsg KO spermatogonia can undergo normal progression to the spermatocyte stage, BSG-mediated germ cell-Sertoli cell interactions appear to be necessary for integrity of the BTB and spermatocyte progression to mature spermatozoa.
